Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Saito, Takumi*; Motokawa, Ryuhei; Okubo, Takahiro*; Miura, Daisuke*; Kumada, Takayuki
Environmental Science & Technology, 57(26), p.9802 - 9810, 2023/07
Times Cited Count:0 Percentile:0(Engineering, Environmental)Kimuro, Shingo*; Kirishima, Akira*; Nagao, Seiya*; Saito, Takumi*; Amano, Yuki; Miyakawa, Kazuya; Akiyama, Daisuke*; Sato, Nobuaki*
Journal of Nuclear Science and Technology, 55(5), p.503 - 515, 2018/05
Times Cited Count:7 Percentile:58.07(Nuclear Science & Technology)no abstracts in English
Ueda, Masato; Sakamoto, Yoshiaki*
Genshiryoku Bakkuendo Kenkyu, 12(1-2), p.31 - 39, 2006/03
no abstracts in English
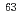 Ni migration through crushed rock media
Ni migration through crushed rock mediaTanaka, Tadao; Sakamoto, Yoshiaki; Mukai, Masayuki; Maeda, Toshikatsu; Nakayama, Shinichi
Radiochimica Acta, 92(9-11), p.725 - 729, 2004/12
Times Cited Count:1 Percentile:10.03(Chemistry, Inorganic & Nuclear)Migration experiments of 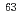 Ni for crushed rocks, granite and tuff, were performed under the coexistent condition with a humic acid and a fulvic acid of 0-30 mg/l in concentration, which are Nordic humic substances supplied from International Humic Substance Society. Migration experiments of Ni had been performed by a column system, to investigate migration behavior of Ni through a column packed crushed rock. The Ni concentration in the effluent passed through the column was corresponding to the fractional percentage of Ni complexing with humic substance in influent solution. This result suggests that the Ni complexing with humic substance in influent solution was flowed out from the column without any effective interactions with the rock media. The migration behavior of Ni could be expressed by a migration model taking account of the complexation kinetics of Ni with humic substance in the aqueous phase.
Ni for crushed rocks, granite and tuff, were performed under the coexistent condition with a humic acid and a fulvic acid of 0-30 mg/l in concentration, which are Nordic humic substances supplied from International Humic Substance Society. Migration experiments of Ni had been performed by a column system, to investigate migration behavior of Ni through a column packed crushed rock. The Ni concentration in the effluent passed through the column was corresponding to the fractional percentage of Ni complexing with humic substance in influent solution. This result suggests that the Ni complexing with humic substance in influent solution was flowed out from the column without any effective interactions with the rock media. The migration behavior of Ni could be expressed by a migration model taking account of the complexation kinetics of Ni with humic substance in the aqueous phase.
Tanaka, Tadao; Sakamoto, Yoshiaki; Sawada, Hiroshi; Ogawa, Hiromichi
JAERI-Conf 2003-010, p.134 - 141, 2003/09
We have performed migration experiments of Np(V) and Am(III) for crushed granite, under the coexistent condition with humic acid substance. As for Np, the periodical concentration changes in the breakthrough curve and the migration velocity of Np passed through the column were not affected by the coexistence of the humic substance. As for Am, on the other hand, the periodical concentration changes in the breakthrough curve were affected by the humic substance concentration. The migration behavior of Am passed through the present column system could be expressed by a migration model taking account of the non-equilibrium state.
Ozaki, Takuo; Ambe, Shizuko*; Abe, Tomoko*; Francis, A. J.
Analytical and Bioanalytical Chemistry, 375(4), p.505 - 510, 2003/02
Times Cited Count:7 Percentile:23.17(Biochemical Research Methods)no abstracts in English
Tanaka, Tadao; Sakamoto, Yoshiaki; Ogawa, Hiromichi
Genshiryoku Bakkuendo Kenkyu, 9(1), p.29 - 34, 2002/09
To obtain structural information on functional groups of humic substances complexing with metal ions, infrared (IR) spectral analysis of the humic substances dissolving in aqueous solutions has been studied by ATR (Attenuated Total Reflection) method. By using the ATR method, IR spectral shifts of humic substance due to complexation with metal ions were observed. The observation provided with information on complexation kinetics, and on characteristics of functional groups depending on solvent properties, such as pH and solute concentrations. These information was difficult to be obtained by conventional FTIR spectroscopy using powdered humic substances. The obtained results show that IR spectral shifts on the functional groups of the humic substances complexed with metal ions could be possibly characterized  by using the ATR-FTIR spectroscopy.
by using the ATR-FTIR spectroscopy.
Nagao, Seiya; Aoyama, Masakazu*; Watanabe, Akira*; Nakaguchi, Yuzuru*; Ogawa, Hiromichi
Analytical Sciences (CD-ROM), 17(Suppl.), p.1585 - 1588, 2002/03
no abstracts in English
Fukushima, Masami*; Tatsumi, Kenji*; Nagao, Seiya
Environmental Science & Technology, 35(18), p.3683 - 3690, 2001/09
Times Cited Count:135 Percentile:92.77(Engineering, Environmental)no abstracts in English
Nagao, Seiya; Nakaguchi, Yuzuru*; Suzuki, Yasuhiro*; Hiraki, Keizo*; Fujitake, Nobuhide*; Ogawa, Hiromichi
Proceedings of International Conference on the Biogeochemistry of Trace Elements (ICOBTE2001), P. 666, 2001/00
no abstracts in English
Sakamoto, Yoshiaki; Nagao, Seiya; Ogawa, Hiromichi; Rao, R. R.*
Radiochimica Acta, 88(9-11), p.651 - 656, 2000/09
Times Cited Count:11 Percentile:59.85(Chemistry, Inorganic & Nuclear)no abstracts in English
Mine, Tatsuya*; Mihara, Morihiro;
JNC TN8430 2000-010, 27 Pages, 2000/07
In the geological disposal system of the radioactive wastes, gas generation by microorganism could be significant for the assessment of this system, because organic material included in groundwater, buffer material and wastes might serve as carbon sources for microorganisms. In this study, gas generation tests using microorganisms were carried out under anaerobic condition. The amount of methane and carbon dioxide that were generated by activity of Methane Producing Bacteria (MPB) were measured with humic acid, acetic acid and cellulose as carbon sources. The results showed that methane was not generated from humic acid by activity of MPB. However, in the case of using acetic acid and cellulose, methane was generated, but at high pH condition (pH=10), the amount of generated methane was lower than at low pH (pH=7). It was not clear whether the pH would affect the amount of generated carbon dioxide.
Tochiyama, Osamu*
JNC TJ8400 2000-044, 53 Pages, 2000/02
To estimate the polyelectrolyte effect and the effect of the heterogeneous composition of humic acids, the complex formation constants of Eu(III) and Ca(II) with Aldrich humic acid and polyacrylic acid were obtained, for Eu(10 to 10
to 10 M) by solvent extraction with TTA and TBP in xylene, for Ca (10
M) by solvent extraction with TTA and TBP in xylene, for Ca (10 M) with TTA and TOPO in cyclohexane and for Ca(10
M) with TTA and TOPO in cyclohexane and for Ca(10 M) by using ion-selective electrode. By defining the apparent formation as
M) by using ion-selective electrode. By defining the apparent formation as  = [MR
= [MR ]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR
]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR ] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log
] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO
have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO and NaCl. Log
and NaCl. Log of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log
of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log
increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log , the decrease in log
, the decrease in log of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log
of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log of polyacrylate, log
of polyacrylate, log of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log
of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log  of humate by 0 to 0.8. The dependence of log
of humate by 0 to 0.8. The dependence of log of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
Ueta, Shinzo*; *; *; *
JNC TJ8400 2000-002, 364 Pages, 2000/02
Japan Nuclear Cycle Development Institute (JNC) have been setting migration parameters and developing its database for the 2nd Progress Report of HLW Geological Disposal (H12 Report). In this study, experimentswere carried out to certify the reliability of parameters and scenario, and examination was carried out to survey procedures of quality management. The main contents are as follows. (1)Data acquisition for certification of migration parameters. The effect of NH complex of Pd on distribution coefficients (Kd) of Pd on both bentonite and rocks, and the effect of sulfate and carbonate complexes of Am on Kds of Am on bentonite are investigated. Kds of Pd depended on NH
complex of Pd on distribution coefficients (Kd) of Pd on both bentonite and rocks, and the effect of sulfate and carbonate complexes of Am on Kds of Am on bentonite are investigated. Kds of Pd depended on NH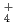 concentration in aqueous. The dependence varied with pH. Effects of sulfate and carbonate complexes on Kds of Am were not remarkable. Apparent diffusivities of Cs in bentonite saturated by saline water were measured. It was confirmed that the apparent diffusivities of Cs in saline water were similar to those in pure water. (2)Evaluation of colloidal effect on nuclide migration. An evaluation of validity of analytical model (Hwang's model) for nuclide migration under existence of colloids and investigation of characterization of colloids in groundwater were carried out. As the results, it was indicated that the Hwang's model was appropriate, and it was found that samplingtechnique influenced concentration and size distribution of colloids. (3)Influence of organic substances on solubility. Solubility of Th was measured under the condition with humic acid and carbonate. It increased roughly in proportion to the concentration of humic acid. And it was remarkably high under the condition with carbonate. It was confirmed that Th solubility data set in H12 report was conservative, even though humic acid existed in groundwater. (4)Use of Mechanistic Models for Safety Assessment. The integrated sorption/diffusion model has been used to calculate K
concentration in aqueous. The dependence varied with pH. Effects of sulfate and carbonate complexes on Kds of Am were not remarkable. Apparent diffusivities of Cs in bentonite saturated by saline water were measured. It was confirmed that the apparent diffusivities of Cs in saline water were similar to those in pure water. (2)Evaluation of colloidal effect on nuclide migration. An evaluation of validity of analytical model (Hwang's model) for nuclide migration under existence of colloids and investigation of characterization of colloids in groundwater were carried out. As the results, it was indicated that the Hwang's model was appropriate, and it was found that samplingtechnique influenced concentration and size distribution of colloids. (3)Influence of organic substances on solubility. Solubility of Th was measured under the condition with humic acid and carbonate. It increased roughly in proportion to the concentration of humic acid. And it was remarkably high under the condition with carbonate. It was confirmed that Th solubility data set in H12 report was conservative, even though humic acid existed in groundwater. (4)Use of Mechanistic Models for Safety Assessment. The integrated sorption/diffusion model has been used to calculate K , D
, D and D
and D values ...
values ...
*; Sato, Haruo; *
JNC TN8400 99-059, 59 Pages, 1999/10
Organic acids in groundwater are considered to form complexes and increase the solubility of radionuclides released from vitrified waste in a high-level radioactive waste (HLW) repository. To ivestigate whether the solubility of samarium (Sm) is influenced by organic substances, we measured Sm solubility in the presence of different organic substances and compared those values with results from thermodynamic predictions. Humic acid (Aldrich) is commercially available and soluble organic matter originated from bentonite were used as organic substances in this study. Consequently, the solubility of Sm showed a tendency to apparently increase with icreasing the concentration of humic acid, but in the presence of carbonate, thermodynamic predictions suggested that the dominant species are carbonate complexes and that the effect of organic substances are less than that of carbonate. Based on total organic carbon (TOC), the increase of Sm solubility measured with humic acid (Aldrich) was more significant than that in the case with soluble organic matter originated from bentonite. Since bentonite is presumed to include also simple organic matters of which stability constant for forming complexes is low, the effect of soluble organic matter originated from bentonite on the solubility of Sm is eonsidered to be less effective than that of humic acid (Aldrieh). Experimental values were compared with model prediction, propsed by Kim, based on data measured in a low pH region. Tentatively we calculated the increase in Sm solubility assuming complexation with humic acid. Trial calculations were carried out on the premise that the complexation reaction of metal ion with humic acid is based on neutralization process by 1-1 complexation. In this process, it was assumed that one metal ion coordinates with one unit of complexation sites which number of proton exchange sites is equal to ionic charge. Consequently, Kim's model indicated that carbonate complexes should be dominant ...
Tanaka, Tadao; Sakamoto, Yoshiaki; Muraoka, Susumu
JAERI-Conf 99-004, p.662 - 673, 1999/03
no abstracts in English
Takahashi, Yoshio*; Kimura, Takaumi; Kato, Yoshiharu; Minai, Yoshitaka*
Environmental Science & Technology, 33(22), p.4016 - 4021, 1999/00
Times Cited Count:46 Percentile:74.45(Engineering, Environmental)no abstracts in English
Takahashi, Yoshio*; Kimura, Takaumi; Kato, Yoshiharu; *; *; *
Journal of Radioanalytical and Nuclear Chemistry, 239(2), p.335 - 340, 1999/00
Times Cited Count:15 Percentile:72.26(Chemistry, Analytical)no abstracts in English